Abstract
Background: In sickle cell disease (SCD), a mutation in the beta globin gene results in an abnormal hemoglobin, HbS, which polymerizes under low oxygen conditions. These hemoglobin polymers impact red cell adhesion, deformability, and whole blood viscosity. For thirty years, there was only one widely used SCD modifying agent, hydroxyurea, which could be monitored by conventional laboratory tests. Now, in this era of three new FDA approved drugs for SCD, and several more in the pipeline, as well as gene-based therapy, we need tools to assess the impact of therapies on red cell function and quality. Microfluidics based devices could meet this need, but there is a lack of robust, easy to use, clinically validated microfluidic devices. Here we describe the collaborative development of a laminin lined microfluidics device intended to evaluate the impact of SCD modifying therapies on red cell adhesion to the microvasculature. Early versions of the device were limited in function due to flawed device design and an inadequate hematocrit and BSA concentration. Prior to optimization, the sample and wash lines shared a single inlet port. The current device separates the two, keeping the wash port independent and permitting a clean wash post sample perfusion. Samples used in development were unaltered; Patient samples were introduced early in development to direct optimization.
Methods: Samples were collected into EDTA coated tubes and stored at 4 degrees C. Samples were spun in a centrifuge at 200g for 15 minutes at 4 degrees C. Plasma and buffy coat layers were removed via aspiration. 500ul of 1X PBS was added to each tube.
Devices were fabricated using polydimethylsiloxane (PDMS). After pouring individual devices, the mold was placed into a desiccator for 1 hour and cured at 60 degrees C overnight. Devices were diced and permanently bonded to seal the microchannels. Devices were stored in a 60 degree C oven.
100ug/ml solution of Human Placental Laminin was prepared. 20ul of laminin was applied to each channel and incubated overnight at 4 degrees C. 25ul of Bovine Serum Albumin was applied to each channel and incubated at room temperature for 2 hours. Channels were washed with 1x PBS.
Samples were transferred to a 1mL syringe; A pre-assembled needle tip with tubing was added to the syringe. Sample tubing was connected to the appropriate inlet port. To visualize adhesion, the device was mounted on top of an inverted bright-field microscope. Harvard Apparatus syringe pump was used to perfuse samples and wash at the relevant flow rate.
Channels were imaged at 20x magnification. Images were taken at the same ROI and adhered cells were counted manually and using proprietary cell quantification software.
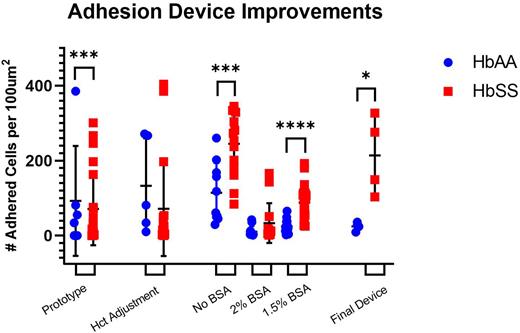
Figure 1: Measurements for adhered RBC and sRBC at sequential stages of development. (a)HbAA and HbSS adhesion captured in current adhesion device (p-value=0.0358) (N=8); (b); HbAA and HbSS adhesion captured at 20% hct. (c) HbAA and HbSS adhesion captured at BSA concentrations. No BSA: (p-value=0.001) (N=24) | 1.5% BSA: (p-value< 0.0001) (N=57)
Conclusion: With use of our robust adhesion device, sRBC function and its correction can be monitored pre-therapy and throughout treatment; Then, make selection of the appropriate second agent based on individual need and drug effect. Our results indicate clear improvements from the prototype to the current version, achieving genotype discrimination with significant difference (p = 0.0358). Due to a limited sample size, results at 20% hct were not significantly different. Under no BSA, separation between HbAA and HbSS was achieved with significance, but an undesirable overlap between the two encouraged continued concentration testing. 2% BSA extensively diminished adhesion among sRBC, reducing adherence to near RBC levels. 1.5% BSA afforded an ideal adhesion range for both HbAA and HbSS (p<0.0001).
Rather than using glyceraldehyde modified RBCs, we incorporated fresh patient samples into the development process to more suitably analyze RBC adherence. With no intent to replicate in vivo conditions, our device was constructed to analyze red cell changes on an individual level. We propose a functional adhesion assay to produce biomarkers that will correlate with SCD clinical course. With analytical and clinical validation, our microfluidic assay can capture functional changes to RBCs and provide objective, quantitative biomarkers of pain events for use in clinical trials and in patient care
Disclosures
Sheehan:Global Blood Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.